Least concern (IUCN; assessed August 29, 2023; population trend, decreasing)
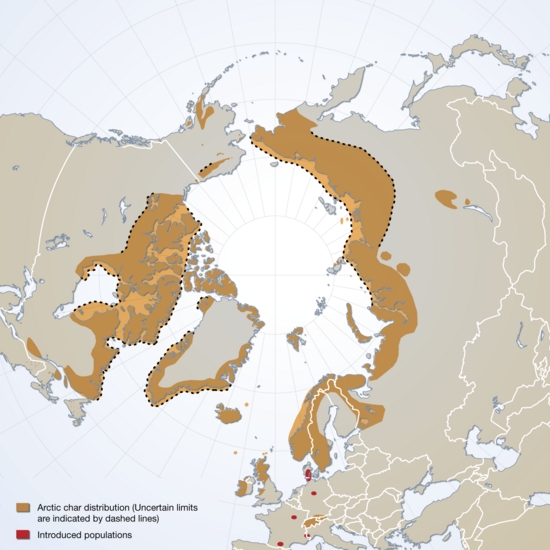
The Arctic char belongs to the genus Salvelinus (which comprises other chars, including the brook and lake “trouts” (Salvelinus fontinalis and Salvelinus namaycush, respectively)). Chars are salmonid fishes (like the true trouts (genus Salmo) and the Pacific salmons (genus Oncorhynchus)), all of which are routinely harvested for food or form the basis for an economically and socially valuable sport and recreational fishing industry. Salmonid fishes can have diverse life histories, even within species, depending on the ecological context in which they find themselves. Arctic char displays these divergent ecological characteristics in full. There are two major ecological types (‘ecotypes’): landlocked, wholly freshwater-inhabiting populations (‘potamodromous’) that complete their reproductive cycles within these confines, and river-run populations (‘anadromous’) in which both reproductive (spawning) and early development (hatching of eggs and maturation of larvae to the ‘smoltification’ stage) occurs within freshwaters, followed by migration of the ‘smolts’ (juvenile char) to saltwater (estuaries and nearshore marine environments) for maturation to full, sexually-mature adults. The adults then migrate the following spring back into freshwaters to spawn, and the cycle begins anew (a reproductive strategy known as ‘iteroparous’ reproduction (some salmonids, such as Pacific salmon, spawn once and die (‘semelparous’ reproduction)). In Arctic rivers, Arctic char undergoes two major migrations. The spring migration sees both adults and newly smelted juveniles swim downstream to the estuarine and coastal marine realm to feast on the bounty of the seas. Here the juveniles mature into adults. Towards the end of summer and beginning of fall, the char migrates back upstream to the rivers in which they were born (aside from a few individuals which may migrate among populations and breed therein, allowing some genetic material to seep between populations).
As mentioned above, the Arctic char is often a key element of Arctic food security for Indigenous populations in Canada, but especially for Inuit in their land across the Arctic and Sub-Arctic (Inuit Nunangat). For example, in Nunavik, this fish is the primary source of country food for Inuit inhabiting this region (the Nunavummiut). However, different populations of char are characterized by varying qualities of its flesh, inculcating regional fisheries with distinct tastes and textures, impacting how favourable the species is to communities. Arctic char is both a source of subsistence nutrition and a target for commercially harvesting. Correspondingly, the Arctic char represents a pivotal component of local mixed economies (subsistence and commercial economies) whereby dollars earned compliments the nutritional and cultural value extracted from the species through individual-based harvesting. As an important source of nutrition, Arctic char tends to accumulate fewer pollutant chemicals and contaminants (such as methylmercury) than other country food items such as lake trout, further establishing this species as a critical component of local food supply and security. In Kuujjuaq, Nunavik, the fish is so important that local aquaculture facilities have been set up to raise fertilized eggs, gathered from wild populations, to provide juvenile fish to seed, and subsequently maintain, new char populations in previously uninhabited streams. In this way, brand new fisheries can be established and maintained to safeguard the provision of this important fish to local communities.
Currently, the species is not afforded any legal protection within Canada. It has yet to be assessed by the Canadian Committee for the Status of Endangered Wildlife in Canada (COSEWIC), although as of 2025, it has been placed in a priority list of species to be evaluated. This is likely because of the looming impact of climate change across its wide distribution. In the most recent assessment (2023) of this species’ global population (IUCN), although it lists the char as being of ‘least concern’ from a conservation standpoint, does acknowledge that population numbers are trending downwards. These chars prefer cool waters for spawning, so a warming aquatic environment will reduce recruitment of larval and juvenile fish into adulthood. It is possible that, whilst more southerly populations contract and may even become locally extinct, the high Arctic may provide a refuge except in the most catastrophic of climatic forecasts. That said, with a warming Arctic comes the increasing likelihood of unpredictable impacts on populations of prey organisms, the spreading of diseases and parasites, and the expansion and establishment, in the Arctic, of non-native species (predators and/or competitors), all of which may negatively influence char population stability and longevity.
Freshwater fish species that are comprised of populations that are physically isolated (e.g., lake populations) and/or show behavioural isolation to some degree (e.g., homing spawning migration or ‘natal philopatry’) are often characterised by high degrees of genetic distinctness; or at least that’s what early genetic studies showed. Genomics data has only further reinforced this characterisation, a feature common amongst salmonid fishes like chars. Arctic Canadian populations display significant levels of population structuring at the genomic level. The pivotal anadromous Arctic char fishery of Nunavik, which includes the waters of eastern and southern Hudson Bay, the Hudson Strait, Ungava Bay, and the Labrador Sea, displays significant degrees of genetic differentiation across this area coinciding with the major marine areas just listed, with shallower levels of differentiation of fish in neighbouring rivers that share common estuaries. This is concordant with the knowledge that, in summer, estuaries contain individuals from different breeding rivers. The same study showed that these fish exhibit adaptation at the genomic level to life in both fresh and marine waters (sea surface and air temperatures, in addition to salinity). In landlocked populations, however, the char can take on different forms depending on the diverse microenvironments in lakes in which individuals grow and feed. In Norwegian lakes, some chars are large and feed on benthic (lake bottom) invertebrates, some are smaller benthic feeders (both these forms are darker coloured), some are lighter coloured plankton feeders, whereas others are larger still, but still lightly and silvery decorated, and predators of fish. Such populations are called polymorphic (‘multiple forms’), but are they genetically distinct? Genomic studies of populations in waterbodies in which the these forms may co-exist confirm that this species can adapt both adaptively through natural selection (showing hard changes at the level of the genome) or by utilising “epigenetic” plasticity, in which the ability to differentiate into different morphs depends on particular environmental cues acting upon biochemical processes present in all individuals in a population (and thus encoded in all genomes) to produce, often startlingly, different results. This same study showed, however, that although morphs initially develop through genomic (epigenetic) plasticity, this divergence becomes hardened and results in fixed genetic changes if the populations subsequently become reproductively isolated. Such studies like these show that genomes are capable of dynamic and subtle changes that result in significant biological change at the level of a functioning organism, as well as wholesale changes that in extremis can include chromosomal rearrangements.
As a result of the advance of modern genomic techniques, genomic information can be utilised to develop tools and to inform co-management policies of the char (and other species); and projects like FISHES ( Fostering Indigenous Small‐scale fisheries for Health, Economy, and food Security - FISHES-project) funded by Genome Canada – ensure that such tools are co-developed with – and for the deployment by – northern communities.
© Arctic Institute of North America | Site & Image Information